
- #Cobalt chloride system in weather indicator devices skin
- #Cobalt chloride system in weather indicator devices trial
#Cobalt chloride system in weather indicator devices trial
#Cobalt chloride system in weather indicator devices skin
As cobalt(II) chloride is a skin sensitiser, take care to avoid skin contact and wash hands well after use.

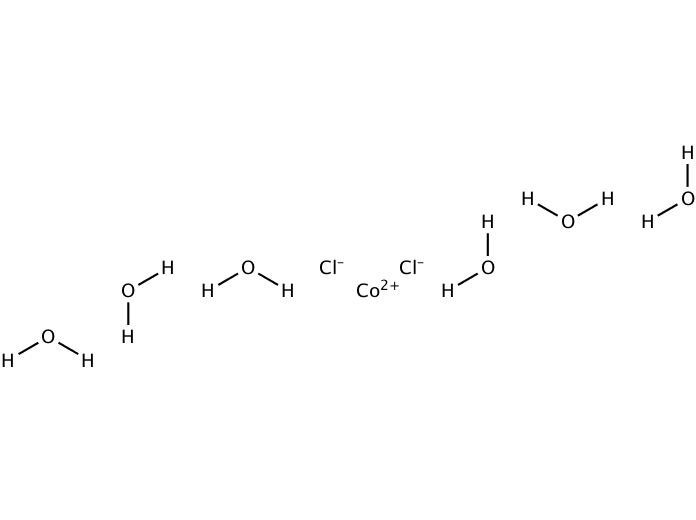
Concentrated hydrochloric acid (CORROSIVE), 100 cm 3.Cobalt(II) chloride-6-water (TOXIC, DANGEROUS FOR THE ENVIRONMENT), 4.0 g.Rack for boiling tubes x1 or x2 (depending on capacity).The demonstration could also be adapted for use as a class experiment with suitable groups. For big groups the reactions should be scaled up, using larger containers such as measuring cylinders or beakers, to improve visibility. Pink cobalt species + chloride ions ⇌ Blue cobalt species + water moleculesĪ white background will help to show the colour changes to best effect. For the purposes of this discussion the equilibrium could adequately be represented by: If students are unfamiliar with the formulae of complex ions this may confuse the issue.

The demonstration can be used to introduce reversible reactions and chemical equilibrium or to illustrate Le Chatelier’s principle once these concepts have been established. The distinctive colours of the two cobalt(II) species in solution produce an attractive visual demonstration of a reversible reaction and the effect of concentration and temperature on the position of equilibrium. The colour changes accompanying the changes in equilibrium position are as predicted by Le Chatelier’s principle. This equilibrium can be disturbed by changing the chloride ion concentration or by changing the temperature.

The two different coloured Co(II) complex ions, 2+ and 2-, exist together in equilibrium in solution in the presence of chloride ions: RSC Yusuf Hamied Inspirational Science Programme.Introductory maths for higher education.The physics of restoration and conservation.


 0 kommentar(er)
0 kommentar(er)
